Abstract
Background: Multiple myeloma (MM) is one of the most common hematological malignancies. The disease is characterized by multiple symptoms resulting from the disease itself, from complications related to therapy, and as a result of the involvement of other organ systems. MM influences various aspects of patient's and family's lives. Therefore, there is a need to better understand the balance between disease control and symptoms management.
Objectives: The main goal of this study is to emphasize the power and importance of Patient Reported Outcome Measures (PROMs) and Family Reported Outcome Measures (FROMs) as additional tools for patient assessment.
This study evaluated the correlation between Patient Reported Outcome Measures (PROMs) and Family Reported Outcome Measures (FROMs) and disease evaluation according to the International Myeloma Working Group (IMW) response criteria in active myeloma patients. A comparison between patient and family reporting (PROMs & FROMs) and the staging of the disease according to the revised international staging system (R-ISS) was done. In addition, this study examined the confounders that may explain the relationship between PROMs and FROMs and disease evaluation.
Methods: This is a quantitative, prospective, observational and longitudinal study of active patients with MM. After receiving Carmel Institutional Review Board approval to conduct the study, we enrolled fifty seven MM patients, the participants completed questionnaires of PROMs and FROMs at intervals of 3 months for one year. In addition, we monitored multiple clinical measures of patient response to treatment.
A descriptive analysis of the research variables has been performed; differences between the PROMs/FROM and clinical variables analyzed by Pearson correlation, comparing PROMs/FROMs mean at the beginning of the study with the results at 3, 6, 9 and 12 months . A mixed regression model was used to examine the predictive ability of the study. In other words, the ability of Patient/Family Reported Outcome to predict the disease evaluation.
Sample size was calculated using Win-Pepi software, using 5% significance and 80% power. For a coefficient of 0.4 between Patient and Family Reported Outcome and MM clinical evaluations, the minimum sample size required is 47 patients, for a coefficient of 0.35, the minimum sample size required is 62 patients. for a coefficient of 0.50, the minimum sample size required is 37 patients. This study recruited a sample size of 57 patients.
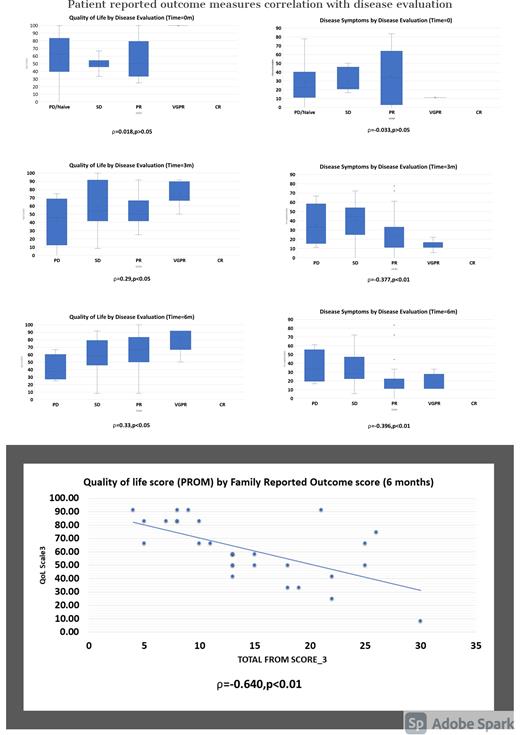
Results - Fifty-seven patients participated in this study. After 3 months of treatment, a better disease evaluation was associated with improvement in disease symptoms or side effects reported by the patient. Furthermore, a better disease response was associated with a better body image scale and better future perspective. We observed a similar association after 6 and 9 months.
In addition, the more the patient reported side effects or disease symptoms, the more it affects the family member (PROMs were positively correlated with FROMs). A better body image and future perspective reported by patient was associated with a lower effect on family member (PROMs were negatively correlated with FROMs)).
A positive significant correlation was found between physician ranking of physical status ECOG (Eastern Cooperative Oncology Group) and the effect on family members. In other words, the worse the physical status of the patient, the more it affect the family member (in months 0,3,6 and 9).
These finding were supported by the mixed model analysis, which showed a significant effect of disease symptoms, appetite loss, physical function, future perspective, and global satisfaction in prediction of clinical status.
Conclusion- There is a significant relation between PROM/FROM and the typical assessment tools. This study highlights the power of PROM/FROM tools to evaluate patient from his point of view and to adjust the treatment accordingly. Finally, this study raises up the importance of continuing the research about the effect on the family member as a result of the patient disease and clinical status.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal